Abstract
CSF3R, the receptor for granulocyte colony stimulating factor, is a critical regulator of neutrophil production. Multiple CSF3R mutants and mRNA splice variants have been identified. CSF3R variant 1 (V1) is the canonical isoform, CSF3R variant 3 (V3) is hypo-proliferative with diminished capacity to support granulopoiesis relative to V1, while CSF3R variant 4 (V4) is hyper-proliferative with the lowest capacity to support neutrophil differentiation. CSF3R mutant variants demonstrate a spectrum of phenotypes, from loss-of-function to ligand-independent autoactivation. All three splice variants are expressed in normal cells and the expression of the isoforms is often altered in myeloid malignancies. Additionally, CSF3R mutations are typically heterozygous, nonetheless CSF3R variants have historically been studied in homo-dimeric model systems. We hypothesized that co-expression of the various CSF3R isoforms and mutants will alter CSF3R function via heterodimerization.
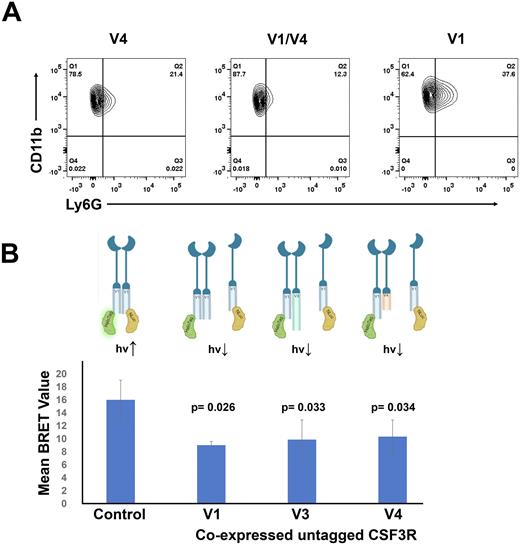
We co-expressed the CSF3R splice variants in both the cytokine dependent BaF3 cell line and ER-HoxB8 cells (conditionally immortalized myeloid progenitor cells) to study proliferation and differentiation. We have shown that co-expression of V1 with V3 partially rescues the hypo-proliferative phenotype and reduced JAK/STAT activation in response to G-CSF stimulation observed in cells expressing V3 alone. Co-expression of V1 with V4 lead to decreased proliferation relative to cells expressing V4 alone. Moreover, we found that as measured by FACS, cells expressing both V1 and V4 demonstrated reduced differentiation with fewer CD11b+/Ly6G+ cells relative to either cells expressing V1 or V4 alone (Figure 1A). Thus, when co-expressed CSF3R splice variants produce altered phenotypes compared to when CSF3R splice variants are expressed alone.
To investigate CSF3R dimerization we used bioluminescence resonance energy transfer (BRET). CSF3R fusion protein vectors were created with a BRET luciferase donor (Nluc) or a BRET HaloTag acceptor (HT) at the c-terminal end of each CSF3R variant, and each of these vectors were expressed in pairs. When the tagged receptors are in proximity, energy transfer between Nluc and HT produces the BRET signal. Donor saturation assays (n =3), where the V1-Nluc was held constant and V1-HT was titrated to excess, showed a hyperbolic BRET response, indicating that the observed BRET was via a specific interaction. BRET demonstrated homodimerization and heterodimerization between each of the splice variants, with each pair producing BRET values greater than 10x background, V1:V1 = 20.2, V1:V3 = 21.07, V1:V4 = 19.44, and V3:V4 = 25.76. Significant decreases in BRET were observed when untagged V1, V3, or V4 were co-expressed with V1-Nluc and V1-HT (Figure 1B), confirming CSF3R splice variant heterodimerization and demonstrating that receptor homodimers could be disrupted.
We next tested if co-expression of wild-type CSF3R with mutant CSF3R would likewise alter the observed phenotype. We found that co-expression of V1 with the auto-activating CSF3R-T618I mutant (T618I) produced a small but statistically significant (p= 0.042) decrease in ligand independent proliferation. To test if CSF3R function could be completely inhibited by interrupting the homodimer, we generated a "dead receptor" that lacked all but 27 amino acids of the cytoplasmic domain. When co-expressed with either V1 or T618I-V1, the dead receptor inhibited ligand-induced (for V1) and ligand independent (for T618I) proliferation by more than 50%. BRET confirmed mutant homodimer formation and WT:mutant heterodimer formation. The T618I homodimer demonstrated higher BRET values (27.9 +/- 3.5) compared to the V1 homodimer (17.9 +/- 4.0). Similar results were observed with mutated splice variants, where co-expression of wild-type splice variants inhibited ligand-independent proliferation of T618I-autoactivated splice variants.
In conclusion, CSF3R forms heterodimers of both splice and mutant variants, and this heterodimerization alters the function of the receptor relative to the corresponding CSF3R homodimer. These data emphasize the functional importance of the altered splice variant expression, provide a new paradigm for differential receptor function via heterodimerization, and highlight the need for improved model systems for drug discovery that more accurately recapitulate the natural setting.
Disclosures
Park:Teva: Consultancy; G1 Therapeutics: Consultancy; Gilead: Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding; Rafael Pharma: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.